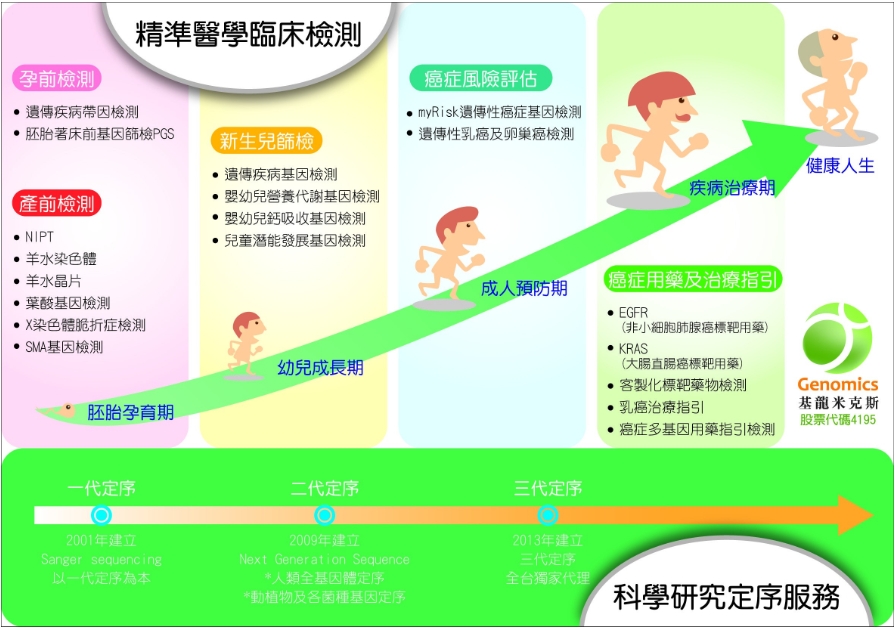
婦幼健康
婦幼健康的市場產值預估超過二十億台幣。基龍米克斯的NIPT檢測是由彰化基督教醫院陳明醫師團隊開發,擁有台灣唯一具有演算法自主專利智財權,更是台灣唯一的NIPT檢測CAP認證實驗室。世界級的品質標準,已逐漸打開市場,年營業額逐年成長。因生活型態的改變,不孕人口增加,每年至少有一萬兩千例試管嬰兒出生。為因應此需求,再度引進彰基開發的『胚胎植入前染色體篩檢(PGS)』技術進入人工生殖的領域;更將陸續增加嬰幼兒營養代謝檢測、兒童潛能基因檢測、遺傳性乳癌及卵巢癌風險評估基因檢測,擴大婦幼健康的產品線。
癌症精準醫學用藥基因檢測
基龍米克斯從2011年即進入標靶藥物基因檢測市場,提供肺腺癌及大腸直腸癌病人組織的標靶藥物基因檢測。2016年積極佈線研發,領先市場成為唯一提供肺腺癌第三代標靶藥物液態活檢T790M基因檢測服務的公司。藉由抽取病人的血液,進行T790M基因檢測確認是否產生突變,可及早搭配使用第三代標靶藥物,有效延長病人存活期。而乳癌已成為國人罹患癌症第三名。化療過程中病患承受的痛苦,讓人對化療望之卻步。基龍米克斯於2016年成立Myriad在臺認證實驗室,提供準確、快速的乳癌基因檢測服務,評估患者未來十年癌症轉移風險,協助醫師和患者評估化療的必要性,此檢測服務已被各大醫學中心醫師採用。目前更積極發展全面性癌症用藥多基因檢測,持續搶進精準醫學基因檢測市場。
基龍米克斯秉持建立定序為本的生技藍海創業理念,領航台灣精準醫學基因檢測市場。在產前基因檢測業績倍數成長下,加碼佈局婦幼健康; 在肺腺癌的標靶藥物液態活檢的領先情勢下,持續投注研發能量於癌症精準醫學用藥基因檢測,全力攻佔倍數增長的醫學基因檢測市場。









